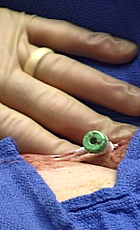
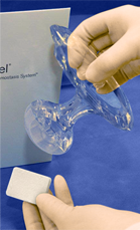
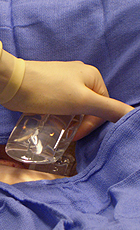
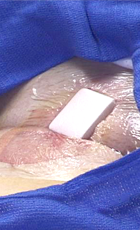
SyvekExcel® – Fast, Easy, Effective Hemostasis For Vascular Catheterization
SyvekExcel utilizes poly-N-acetyl glucosamine fibers derived from microalgae to assist in obtaining and maintaining hemostasis.
- Proven results in ACTs up to 3001
- No foreign materials remain at puncture site2
- Reduced time to ambulation3